Alyssa Hasty, Cornelius Vanderbilt Professor of Molecular Physiology and Biophysics, specializes in immunometabolism, specifically on the role that the immune system plays in obesity and metabolic disease. Recent work from her lab explored the changes in immune cell populations in fat during obesity, weight loss, and weight cycling. The work, led by recent Ph.D. graduate Matthew Cottam and aided by postdoctoral fellow Heather Caslin, was published in Nature Communications in May.
We sat down with Caslin to learn more about the project.
What issue/problem does your research address?
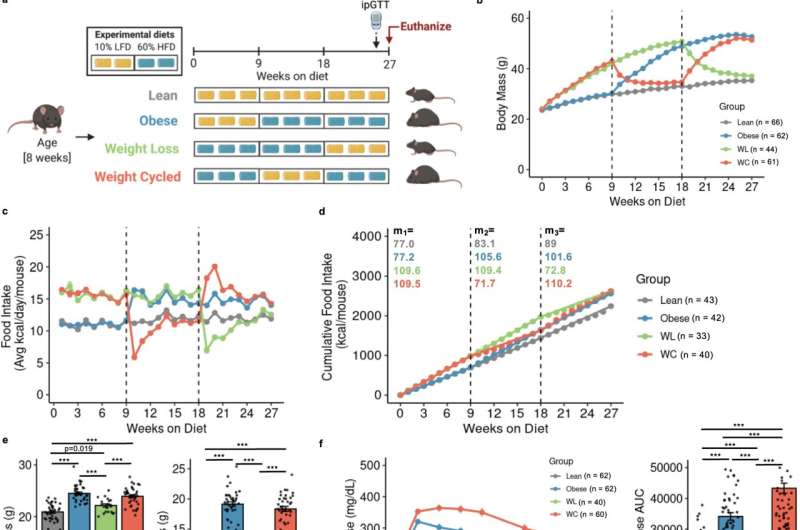
Weight loss is hard to maintain, and many individuals regain lost weight within a few years. Unfortunately, weight cycling—the process of losing and then regaining weight—is a greater diabetes risk than obesity itself. We know that adipose immune cells contribute to obesity-related disease risk, but less is understood about the role of adipose immune cells in weight cycling.
What was unique about your approach to the research?
We used single-cell sequencing, which provides high-resolution information about cellular differences and an improved understanding of an individual cell’s function given its specific microenvironment. We analyzed biological replicates by giving each one its own barcode (tagging each one with antibodies with unique sequences of short segments of DNA) and marked the different cell types within each replicate with other antibodies with unique DNA sequences.
Additionally, Matt created an open-access interactive website called MAIseq, which stands for Murine Adipose Immune sequencing, to facilitate discovery and broaden data accessibility for the scientific community.
What were your findings? How can non-expert readers understand their significance?
We used mice undergoing weight loss and regain to understand how weight cycling worsens diabetes risk. We found that while weight loss improves blood glucose and reduces diabetes risk, immune cells in the fat remain inflammatory, as they are in obesity, and do not return to their lean state. We believe that the adipose immune cells may “remember” obesity and contribute to the increased diabetes risk observed upon weight regain.
What do you hope will be achieved with the research results in the short and long terms?
Our mouse model mimics human data in that weight cycling increases diabetes risk compared with stable weight obesity. In the short term, we hope to identify specific cell populations that drive the risk increase in our weight-cycling animals. We hope that in the long term this will be translated to human research, providing a mechanism to identify and treat individuals who experience obesity and weight-cycling.
What are the benefits of this research?
If we can pharmacologically target adipose immune cells, we may be able to reduce diabetes risk following weight regain. Additionally, the open-access website created from this research provides a resource for other investigators studying this area.
Where is this research taking you next? What will you personally be doing, or how will other researchers build on this work?
We are currently working to understand how weight cycling impacts T cells, macrophages, and mast cells—different types of immune cells—in the adipose tissue and how these cells contribute to weight cycling-associated diabetes development. Additionally, we are working to understand how weight cycling affects insulin signaling and production.
The paper “Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice” was published in Nature Communications in May 2022.
Matthew A. Cottam et al, Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice, Nature Communications (2022). DOI: 10.1038/s41467-022-30646-4
Citation:
Weight cycling increases diabetes risk (2022, July 20)
retrieved 25 July 2022
from https://medicalxpress.com/news/2022-07-weight-diabetes.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Source link




